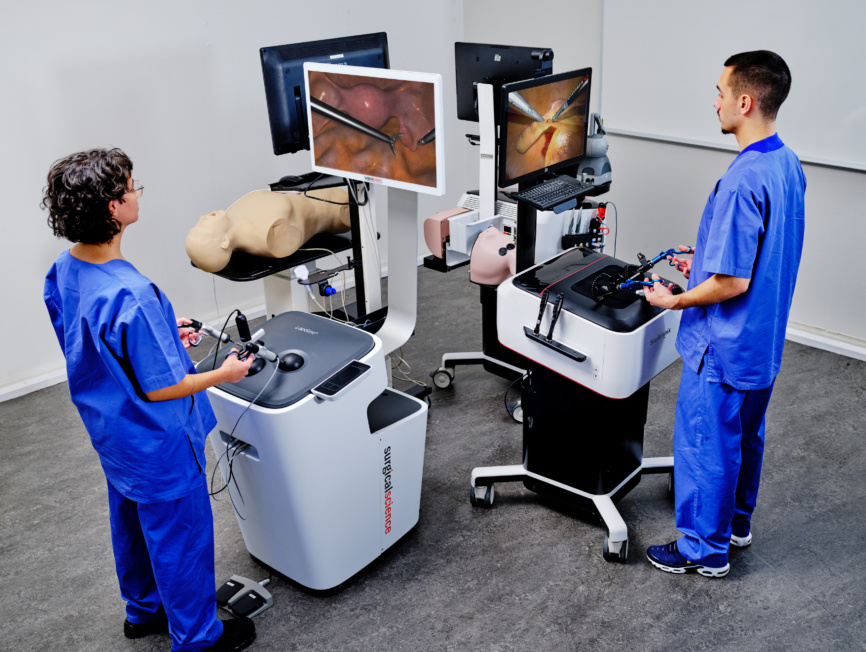
Download Our Product Overview Brochure
Get more information on our full range of simulators.
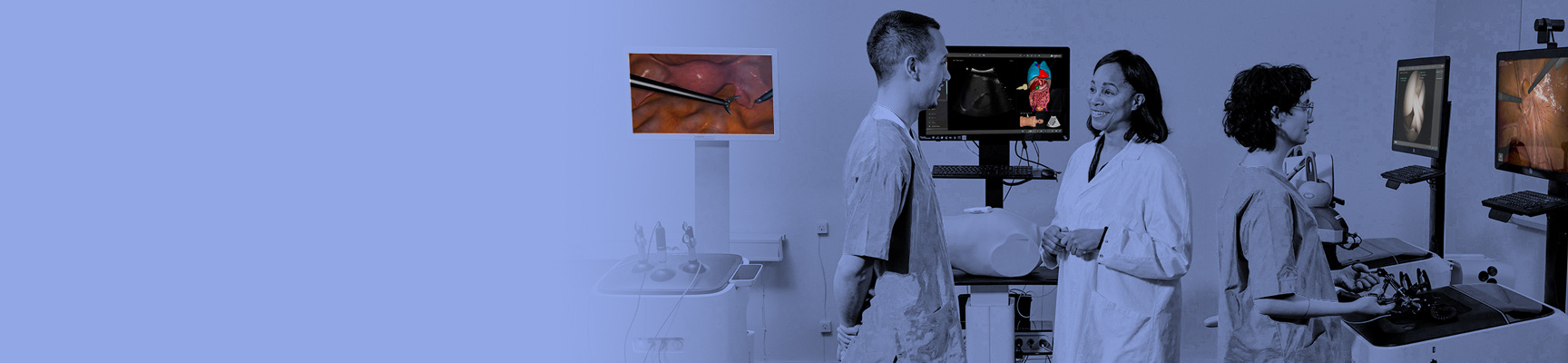
Our line of simulator products offers powerful technical training for healthcare professionals. Developed in collaboration with medical associations worldwide, it is a robust platform to learn and master critical skills to ensure procedural efficiency and promote quality outcomes. Find your simulator by focus area, or via the button:

Get more information on our full range of simulators.